The Challenge
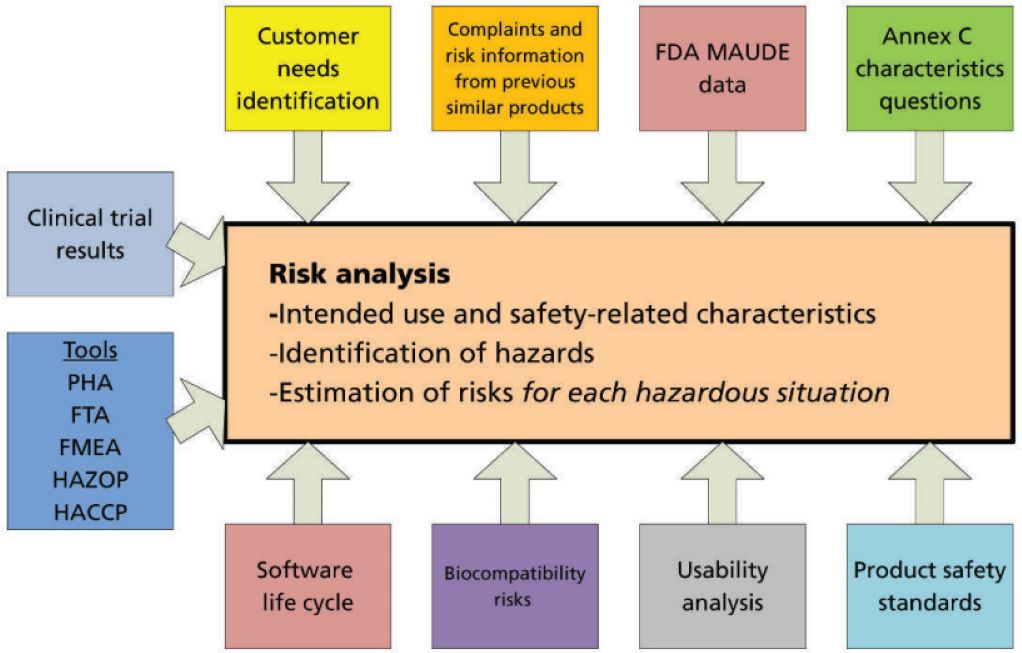
In today’s highly regulated healthcare landscape, medical device manufacturers face mounting pressure to ensure patient safety, regulatory compliance, and product reliability. A single oversight in risk management can trigger severe consequences—ranging from costly recalls and reputational damage to regulatory penalties and, most importantly, patient harm. The challenge is compounded by the fact that risk management is not a one‑time activity but a continuous process that must be embedded into every stage of the product lifecycle. Standards such as ISO 13485:2016 require documented processes for risk management, while the FDA’s Quality System Regulation (21 CFR Part 820) and Europe’s MDR 2017/745 mandate rigorous oversight of design controls, post‑market surveillance, and risk mitigation. With global regulatory bodies tightening their expectations, manufacturers need a partner who not only understands the complexities of applying risk management per the ISO 14971:2019 but can also streamline the process for long‑term success.
Why FDAmedix
FDAmedix has built its reputation as a trusted leader in medical device risk management by combining deep regulatory expertise with practical industry experience. We help manufacturers identify, assess, and mitigate risks at every stage of the product lifecycle, from concept and design through production and post‑market monitoring. By tailoring strategies that align with ISO 14971:2019, we reduce operational burdens on your team while ensuring your devices meet the highest standards of safety and compliance.

Proven Value
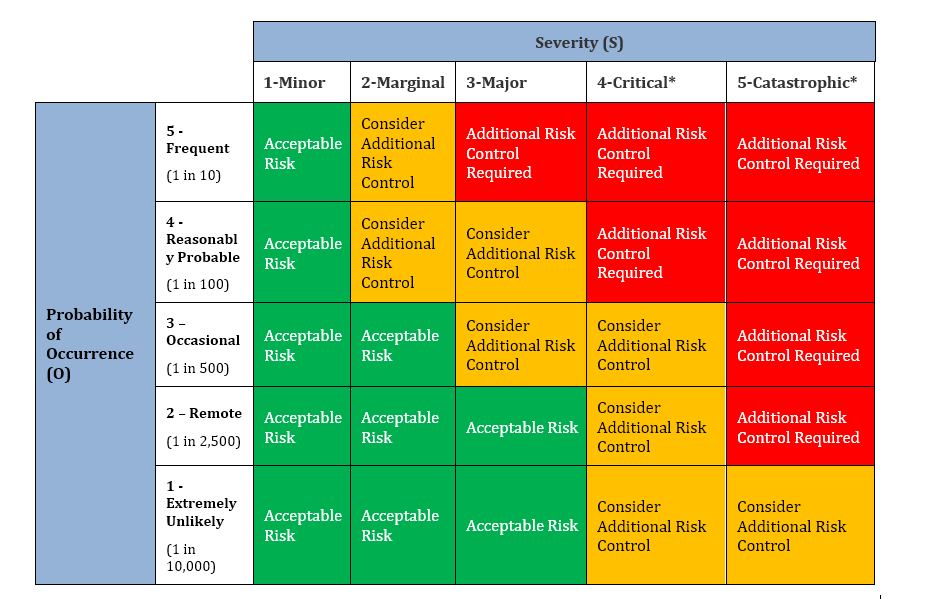
What sets FDAmedix apart is our ability to transform compliance into a competitive advantage. By implementing robust, standards‑driven risk management frameworks, we not only help you meet regulatory expectations but also enhance product quality, accelerate time‑to‑market, and strengthen your reputation with regulators, healthcare providers, and patients. Our clients consistently report fewer audit findings under 21 CFR Part 820, smoother CE marking submissions under the MDR 2017/745, and stronger market positioning in regions that require ISO 13485 certification. Beyond compliance, our approach fosters innovation by reducing uncertainty, enabling your teams to focus on product development while we ensure that risk management is seamlessly integrated into your quality management system.
Comprehensive Support
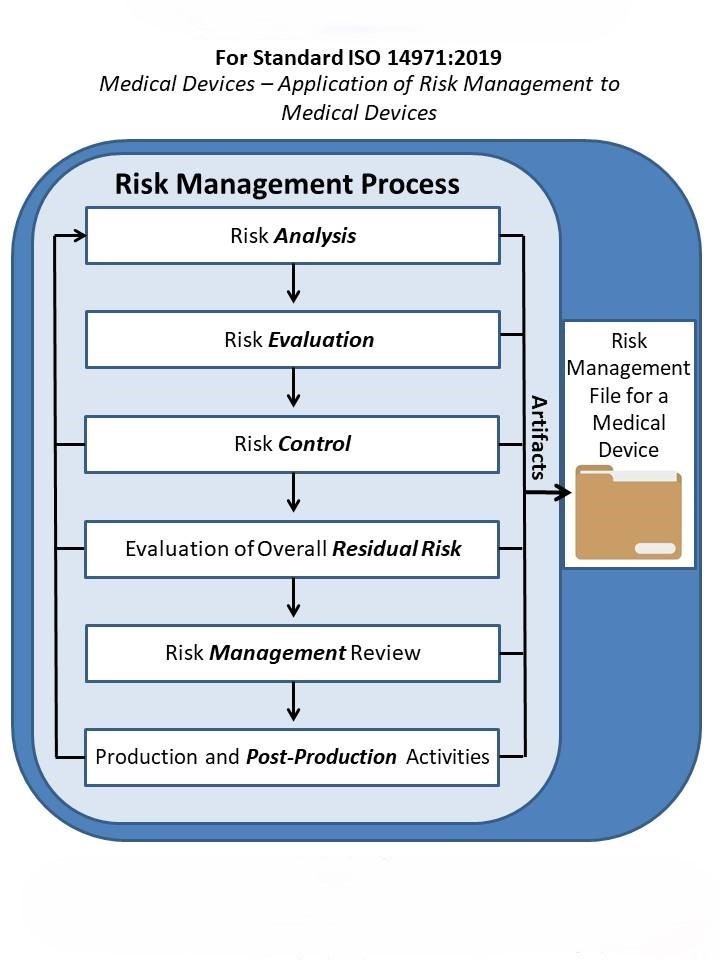
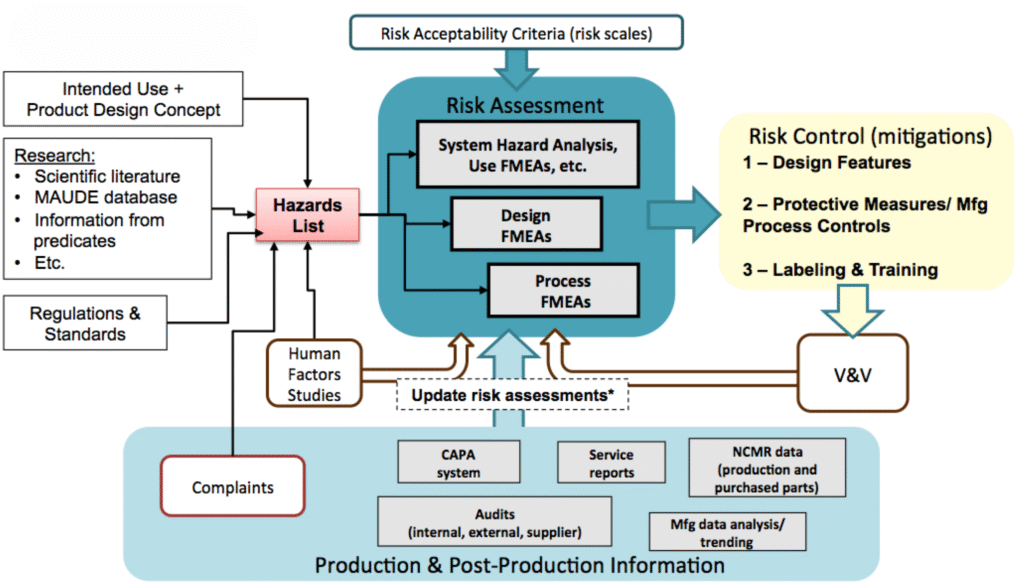
Whether you’re a startup bringing your first device to market or an established manufacturer managing a complex global portfolio, FDAmedix offers scalable solutions to fit your needs. Our services include risk analysis workshops, documentation support, hazard identification, FMEA facilitation, and the implementation of ongoing monitoring systems that align with ISO 13485’s requirements for continual improvement. We also provide guidance on integrating risk management into design controls as required by 21 CFR Part 820, and on meeting the EU MDR’s stringent expectations for and post‑market surveillance. With our guidance, your team can focus on innovation and growth while we ensure your risk management practices are bulletproof, defensible, and globally compliant.
FDAmedix Can Help
In a world where patient safety and compliance are non‑negotiable, risk management is not just a regulatory requirement—it is a business imperative and a driver of long‑term success. Partner with FDAmedix to safeguard your devices, protect your reputation, and unlock new opportunities for growth in global markets. With our expertise in ISO 14971, ISO 13485:2016, 21 CFR Part 820, and EU MDR 2017/745, we help you turn risk into resilience and compliance into competitive advantage. Contact us today to learn how we can strengthen your risk management strategy.