Remote FDA QSR, MDSAP & ISO 13485 Audit Support
We have helped hundreds of organizations achieve certification and compliance to ISO 13485, FDA’s QSR, European Medical Device Directives/Regulations, and Medical Device Single Audit Program (MDSAP).
How We Help
Choose from a full range of medical device audit services
Need an audit done remotely? We can do that.
Baseline or gap assessment
Determine your current level of compliance to relevant quality requirement including FDA cGMPs, ISO 13485, and/or CMDR. We typically conduct a gap analysis before you implement your quality system to identify areas of deficiency.
Pre-assessment audits
Usually conducted 4-8 weeks prior to an official audit, pre-assessment audits occur at the end of an implementation program to identify any remaining weaknesses in a system. Consultants who were not part of the implementation team will identify gaps you need to address and offer guidance for taking effective corrective action.
FDA mock audits
Test your internal systems and personnel in preparation for an FDA inspection. By using Quality System Inspection Techniques (QSIT), Oriel STAT A MATRIX auditors can act as FDA inspectors and assess your cGMP, GLP, GCP, and QSR processes. We can also share techniques for efficiently organizing FDA audits and managing multiple inspectors.
Internal audits
Internal audits are essential to your continuous improvement process and are required by FDA, CE Marking, and other regulations. Oriel STAT A MATRIX offers internal audit services that go beyond the basics to give you higher-level management advice for improving your quality management system and business processes.
Subcontractor or supplier audits
FDA-regulated manufacturers often rely on a complex network of suppliers. A strong supplier audit program will help you manage this network with greater confidence. Oriel STAT A MATRIX’s experienced auditors can verify that your suppliers are meeting relevant requirements, and we can help you create a supplier development program to support suppliers’ corrective action and process improvement efforts.
Auditor training
For medical device professionals, we offer interactive Exemplar (formerly RABQSA)-certified Lead Auditor Training for ISO 13485, Exemplar-certified Internal Auditor Training for ISO 13485 and Internal Auditing to MDSAP training – the first and only MDSAP auditor training offering the opportunity to earn Exemplar Global’s QM-MDSAP Competency certification. Our training, presented largely through hands-on workshops, teaches you the skills you need to plan, conduct, and follow up on compliance audits, as well as how to develop an effective auditing system.
Advanced lead auditor training and implementation
Oriel STAT A MATRIX developed the world’s first performance based auditor training and consulting methodology, which raises the bar in the areas of internal and supplier audits. This innovative approach emphasizes a comprehensive approach to audit preparation and audit execution.
Training Courses
Classes are available as in-person or virtual instructor-led trainings. Select a course to learn more or to register.
| Course Name | Public | Private |
|---|---|---|
| New! Inspection and Audit Readiness Training for Medical Device Manufacturers |  | |
| New! Inspection and Audit Readiness Training for Medical Device Manufacturers |  |
Related Downloads
Provided below are a variety of practice related white papers, market updates and case studies.
| Increasing the Value of Audit Programs with Performance Based Auditing | |
| Becoming an Effective ISO 13485:2016 Auditor 101 |

Achieve and Maintain Regulatory Excellence: The Strategic Partner for Your Remote Medical Device Audits
In the relentlessly evolving medical device landscape, compliance isn’t just a requirement—it’s the foundation of your success. Whether you’re preparing for certification, a regulatory inspection, or optimizing your Quality Management System (QMS), the journey is complex. At [Your Company Name], we move beyond simple auditing. We provide strategic, remote audit solutions that deliver clarity, drive improvement, and build confidence at every stage of your product’s lifecycle.
The Remote Audit Advantage
Strategic Partnership, Not Just a Service:
We don’t just find problems; we provide solutions. Our auditors act as an extension of your team, offering expert insights and actionable recommendations that go beyond a checklist.
Unparalleled Flexibility and Efficiency:
Reduced Costs: Eliminate travel expenses for both your team and auditors, allowing you to invest your resources where they matter most.
Minimal Disruption: Conduct your audit without impacting your daily operations. Your team stays productive, and your manufacturing lines keep running.
Faster Scheduling: Our agile, technology-driven process eliminates travel logistics, allowing for more flexible scheduling and quicker turnaround times.
Secure & Transparent Process: Utilize our secure, encrypted platforms for documentation, interviews, and live facility walk throughs. You maintain full visibility and control throughout the entire process.
Global Reach: Access our expert auditors from anywhere in the world, transcending geographical barriers and time zones.
On-Demand Expertise: Seamlessly bring in specialized subjectmatter experts for specific parts of the audit without the added cost and hassle of travel.
Hybrid Solutions: Benefit from a fully remote process or a hybrid model that blends on-site visits with virtual assessments for maximum flexibility.
Faster Scheduling: Our agile, technology-driven process eliminates travel logistics, allowing for more flexible scheduling and quicker turnaround times.


Our Process: Seamlessly Digital
Kick-off Meeting: We begin with a virtual meeting to define the scope, confirm technology protocols, and create a secure communication plan.
Secure Document Review: You provide access to your quality management system (QMS), electronic records, and other documentation via a secure, encrypted portal.
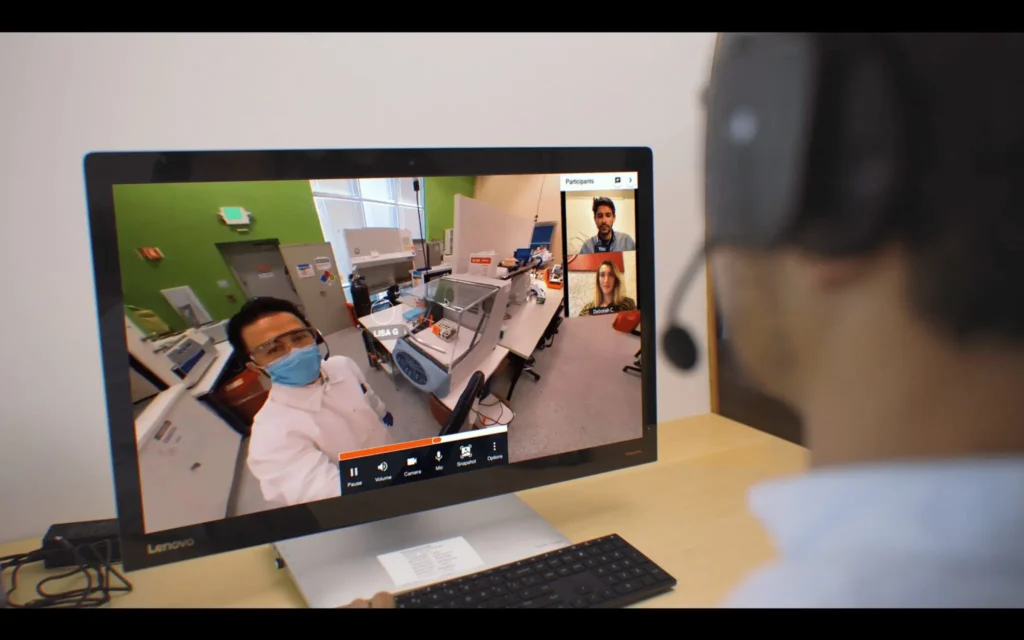
Live Virtual Walkthroughs: Using live video streams (e.g., via webcam or smart glasses), we conduct virtual tours of your manufacturing facilities and observe key processes in real-time.
Remote Interviews: We conduct live, secure interviews with your key personnel to review processes, discuss documentation, and verify compliance.
Closing Meeting & Report: All findings are discussed in a virtual closing meeting, followed by the delivery of a comprehensive, professional audit report.
Remote Audit Types We Can Help You With
- Baseline & Gap Assessments: Identify critical vulnerabilities and opportunities for improvement before they escalate. We provide a clear roadmap to bridge the gap between your current QMS and requirements for ISO 13485, EU MDR/IVDR, or FDA regulations.
- Pre-Assessment Audits: Enter your official audits with confidence. Our experienced auditors evaluate your systems and processes, pinpointing areas of non-conformance and preparing your team to address questions effectively.
- Mock FDA Inspections: Simulate a real FDA inspection, right down to the interview process, using the FDA’s Quality System Inspection Technique (QSIT). This risk-free practice run uncovers weaknesses, boosts staff confidence, and helps you correct issues before an official inspection, saving you from costly 483 observations or warning letters.
- ISO 13485 Audits: We verify your QMS’s conformity to the international standard for medical device quality management. Our remote audits offer a cost-effective and efficient way to prepare for or maintain your ISO 13485 certification.
- EU MDR Audits: Transitioning from the Medical Device Directive (MDD) to the MDR is a significant undertaking. We conduct remote gap analyses and pre-assessment audits to ensure your technical documentation, clinical evaluation reports, and QMS are ready for the rigorous EU regulations.
- Subcontractor & Supplier Audits: Your compliance is only as strong as your supply chain. We perform remote audits of your critical suppliers and subcontractors to ensure their QMS and processes meet your quality and regulatory standards. This reduces your internal costs and provides peace of mind.
Contact us today for a free consultation and personalized quote.
[Large Call-to-Action Button: “Build Your Compliance Strategy”]