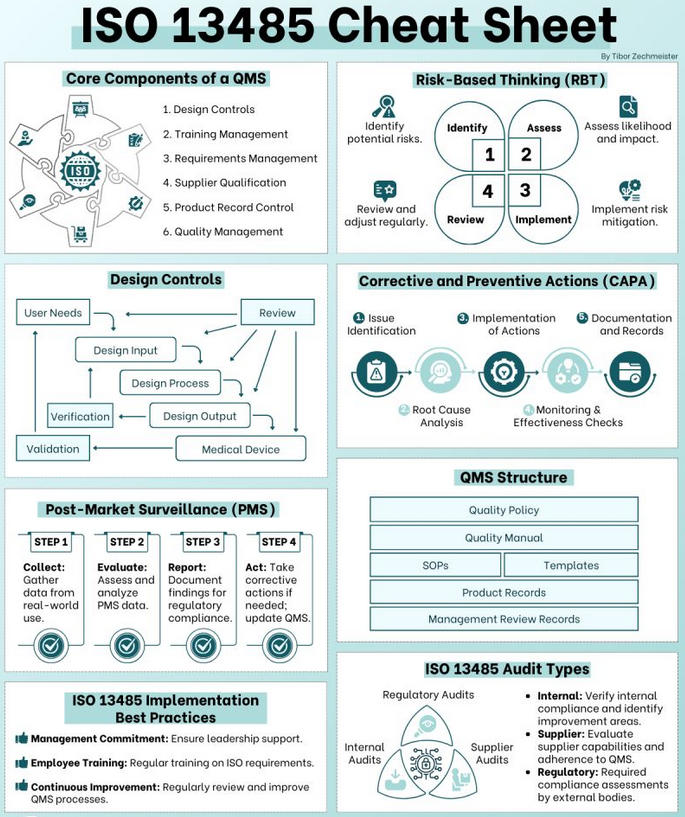
FDA QSR, MDSAP & ISO 13485 Audit Support
We have helped hundreds of organizations achieve certification and compliance to ISO 13485, FDA’s QSR, European Medical Device Directives/Regulations, and Medical Device Single Audit Program (MDSAP).
How We Help
Choose from a full range of medical device audit services
Need an audit done remotely? We can do that.
Baseline or gap assessment
Determine your current level of compliance to relevant quality requirement including FDA cGMPs, ISO 13485, and/or CMDR. We typically conduct a gap analysis before you implement your quality system to identify areas of deficiency.
Pre-assessment audits
Usually conducted 4-8 weeks prior to an official audit, pre-assessment audits occur at the end of an implementation program to identify any remaining weaknesses in a system. Consultants who were not part of the implementation team will identify gaps you need to address and offer guidance for taking effective corrective action.
FDA mock audits
Test your internal systems and personnel in preparation for an FDA inspection. By using Quality System Inspection Techniques (QSIT), Oriel STAT A MATRIX auditors can act as FDA inspectors and assess your cGMP, GLP, GCP, and QSR processes. We can also share techniques for efficiently organizing FDA audits and managing multiple inspectors.
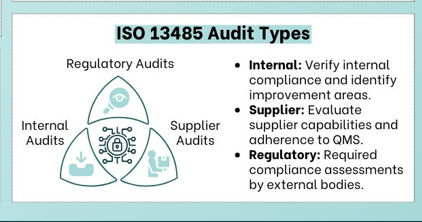
Internal audits
Internal audits are essential to your continuous improvement process and are required by FDA, CE Marking, and other regulations. Oriel STAT A MATRIX offers internal audit services that go beyond the basics to give you higher-level management advice for improving your quality management system and business processes.
Subcontractor or supplier audits
FDA-regulated manufacturers often rely on a complex network of suppliers. A strong supplier audit program will help you manage this network with greater confidence. Oriel STAT A MATRIX’s experienced auditors can verify that your suppliers are meeting relevant requirements, and we can help you create a supplier development program to support suppliers’ corrective action and process improvement efforts.
Auditor training
For medical device professionals, we offer interactive Exemplar (formerly RABQSA)-certified Lead Auditor Training for ISO 13485, Exemplar-certified Internal Auditor Training for ISO 13485 and Internal Auditing to MDSAP training – the first and only MDSAP auditor training offering the opportunity to earn Exemplar Global’s QM-MDSAP Competency certification. Our training, presented largely through hands-on workshops, teaches you the skills you need to plan, conduct, and follow up on compliance audits, as well as how to develop an effective auditing system.
Advanced lead auditor training and implementation
Oriel STAT A MATRIX developed the world’s first performance based auditor training and consulting methodology, which raises the bar in the areas of internal and supplier audits. This innovative approach emphasizes a comprehensive approach to audit preparation and audit execution.
Training Courses
Classes are available as in-person or virtual instructor-led trainings. Select a course to learn more or to register.
| Course Name | Public | Private |
|---|---|---|
| New! Inspection and Audit Readiness Training for Medical Device Manufacturers |  | |
| New! Inspection and Audit Readiness Training for Medical Device Manufacturers |  |
Related Downloads
Provided below are a variety of practice related white papers, market updates and case studies.
| Increasing the Value of Audit Programs with Performance Based Auditing | |
| Becoming an Effective ISO 13485:2016 Auditor 101 |
Oriel STAT A MATRIX’s Goal
Help our life science customers meet regulatory requirements, boost efficiency, and improve patient outcomes REQUEST A PROPOSAL Or ask a question!
Get answers right now. Call

US OfficeWashington DC
EU OfficeCork, Ireland
How We Help
We have helped hundreds of organizations achieve certification and compliance to ISO 13485, FDA’s QSR, European Medical Device Directives/Regulations, and Medical Device Single Audit Program (MDSAP).
Baseline or gap assessment
Determine your current level of compliance to relevant quality requirement including FDA cGMPs, ISO 13485, and/or CMDR. We typically conduct a gap analysis before you implement your quality system to identify areas of deficiency.
Pre-assessment audits
Usually conducted 4-8 weeks prior to an official audit, pre-assessment audits occur at the end of an implementation program to identify any remaining weaknesses in a system. Consultants who were not part of the implementation team will identify gaps you need to address and offer guidance for taking effective corrective action.
FDA mock audits
Test your internal systems and personnel in preparation for an FDA inspection. By using Quality System Inspection Techniques (QSIT), Oriel STAT A MATRIX auditors can act as FDA inspectors and assess your cGMP, GLP, GCP, and QSR processes. We can also share techniques for efficiently organizing FDA audits and managing multiple inspectors.