ISO 13485 Certification Consulting for Medical Device and IVD Manufacturers
ISO 13485 Certification Consulting for Medical Device and IVD ManufacturersFDAmedix is QA/RA consulting, auditing and training firm specializing in regulatory compliance for medical devices and IVDs products. Manufacturers look to us to help them understand or comply with US FDA, EU and international quality system regulation (QSR).
Our team is certified by ISO, FDA, and other global organizations, ensuring that our services meet the highest standards. Whether you’re a multinational corporation or a growing startup, we provide tailored services to meet your unique needs. We provide everything you need to enhance your operations and stay compliant with regulations.
Our team brings decades of combined experience. We hold certifications from ISO, FDA, and other global regulatory bodies, ensuring that our audits meet the highest standards of quality and compliance.”
How We Help
ISO 13485 Certification Opens the Door to Markets Worldwide
We deliver a harmonized QMS that meets US FDA, European, and other international requirements, including those of Australia, Brazil, Canada, and Japan. That’s important, because if your goals include selling outside the US and Europe, ISO 13485 is essential. The QMS we build for you will be ready to expand with you.
Let our QMS team answer these vital questions for you:
- What is ISO 13485:2016 certification?
- How do ISO 13485 and FDA regulations overlap?
- What’s involved in implementing a new QMS?
- How much does it cost to get certified to ISO 13485?
ISO 13485 Certification Opens the Door to Markets Worldwide
We deliver a harmonized QMS that meets US FDA, European, and other international requirements, including those of Australia, Brazil, Canada, and Japan. That’s important, because if your goals include selling outside the US and Europe, ISO 13485 is essential. The QMS we build for you will be ready to expand with you.
Let Our Experienced ISO 13485 Consultants Help You Achieve Certification
We have worked with thousands of medical device and IVD companies, from startups to multinationals. Experience has taught us that a “one size fits all” approach simply does not work. That’s why we take a flexible, staged approach to ISO 13485 implementation, molded around your existing processes, internal resources, and business needs.
What distinguishes our approach is our focus on performance and conformance. We analyze your processes with the goal of improving them before they are standardized and documented. This approach may require some effort and planning at the outset, but the modest investment of time will pay off with improved organizational performance and tangible ROI. Our approach also maximizes your chances of attaining certification during a third-party ISO 13485 audit!
Already Have a QMS That Complies With FDA Regulations?
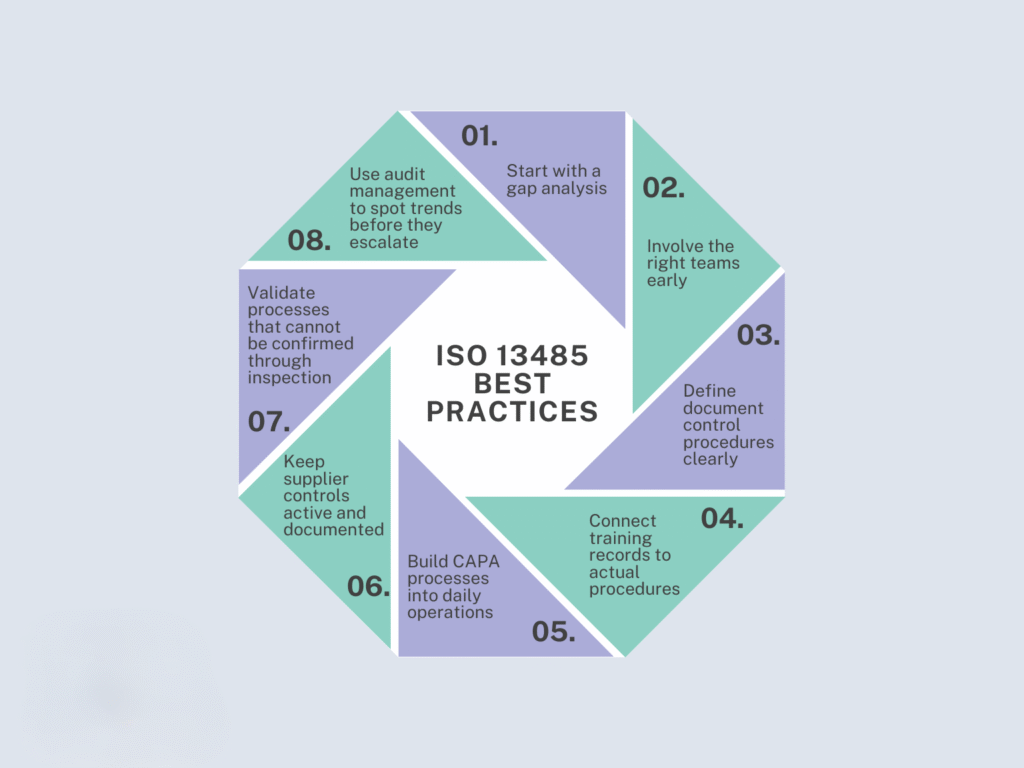
If you have a quality system that complies with FDA 21 CFR Part 820, we will first conduct a gap analysis to assess your current level of compliance with ISO 13485. This analysis will uncover opportunities for improvement and reveal how much time and resource effort will be required to merge ISO 13485:2016 requirements with your existing QMS. We will solidify the path forward as you prepare for your initial ISO 13485:2016 certification audit.
Let’s Get Started!
There’s no time like the present to begin your journey toward ISO 13485 certification. Our QMS implementation and auditing teams are located in the US, Europe, Asia, and Latin America, so we have the resources to get started right away. Give us a call so we can learn more about your needs.
Related Downloads
Provided below are a variety of practice related white papers, market updates and case studies.
ISO 13485 Certification Opens the Door to Markets Worldwide
ISO 13485 is the de facto standard for medical device quality management systems (QMS). We have been helping companies implement, audit, and get trained on ISO 13485 since it was introduced in 1996. Regardless of whether you are formalizing your first QMS or upgrading from FDA QSR, our phased approach will put you on the path to ISO 13485 certification.

Let our QMS team answer these vital questions for you:
How much does it cost to get certified to ISO 13485?
What is ISO 13485:2016 certification?
How do ISO 13485 and FDA regulations overlap?
What’s involved in implementing a new QMS?
ISO 13485 Certification Opens the Door to Markets Worldwide
We deliver a harmonized QMS that meets US FDA, European, and other international requirements, including those of Australia, Brazil, Canada, and Japan. That’s important, because if your goals include selling outside the US and Europe, ISO 13485 is essential. The QMS we build for you will be ready to expand with you.

Let Our Experienced ISO 13485 Consultants Help You Achieve Certification
We have worked with thousands of medical device and IVD companies, from startups to multinationals. Experience has taught us that a “one size fits all” approach simply does not work. That’s why we take a flexible, staged approach to ISO 13485 implementation, molded around your existing processes, internal resources, and business needs.
What distinguishes our approach is our focus on performance and conformance. We analyze your processes with the goal of improving them before they are standardized and documented. This approach may require some effort and planning at the outset, but the modest investment of time will pay off with improved organizational performance and tangible ROI. Our approach also maximizes your chances of attaining certification during a third-party ISO 13485 audit!
Already Have a QMS That Complies With FDA Regulations?
If you have a quality system that complies with FDA 21 CFR Part 820, we will first conduct a gap analysis to assess your current level of compliance with ISO 13485. This analysis will uncover opportunities for improvement and reveal how much time and resource effort will be required to merge ISO 13485:2016 requirements with your existing QMS. We will solidify the path forward as you prepare for your initial ISO 13485:2016 certification audit.

ISO 13485:2016 Consulting and Gap Analysis
Navigating the complexities of ISO 13485 for medical device compliance can be a formidable challenge, especially for small to medium-sized enterprises. Recognizing this, our team has honed an exceptional blend of professional consulting services, meticulously tailored for businesses navigating ISO 13485’s unique demands. Our team of seasoned experts will be your guide through each phase, ensuring the process is clear and manageable. We offer more than just consultancy; we establish a partnership, committed to facilitating a seamless, effective journey to achieving and maintaining ISO 13485 standards. Together, let’s embark on a path to operational excellence.
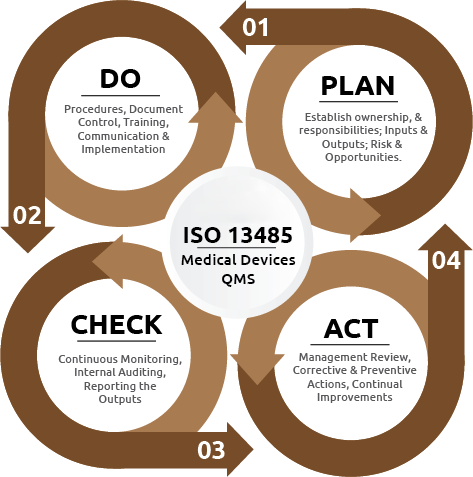
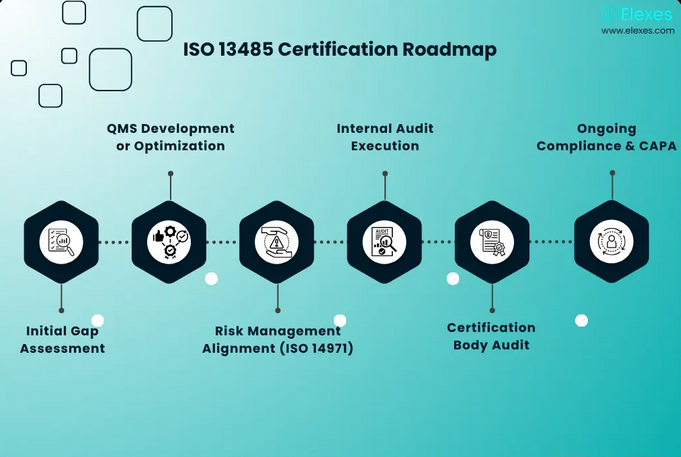
How Do Our Experienced ISO 13485 Consultants Help You Achieve And Maintain Certification?
FDAmedix is a full service ISO 13485 consulting organization. We provide specialized expertise and guidance to organizations seeking certification or compliance with the ISO 13485 standard, which pertains to quality management systems for medical devices. Here are some of the key services that we provide to our clients:
- Gap Analysis: We conduct an initial assessment of the organization’s existing quality management system (QMS) to identify gaps and areas that need improvement to meet ISO 13485 requirements.
- Development of Documentation: We assist in developing and implementing the necessary documentation and procedures required by ISO 13485, including quality manuals, procedures, work instructions, and forms.
- Training and Education: We provide training to employees at all levels of the organization on ISO 13485 requirements, QMS principles, and best practices for compliance. This may include training on documentation procedures, internal auditing, and continual improvement processes.
- Internal Audits: FDAmedix conducts internal audits of the organization’s QMS to assess compliance with ISO 13485 requirements and identify areas for improvement. We also assist in developing and implementing corrective and preventive actions (CAPAs) to address non-conformities.
- Preparation for Certification Audits: We prepare the organization for external certification audits by helping to ensure that all necessary documentation and processes are in place and that employees are adequately trained and prepared for the audit.
- Continual Improvement: FDAmedix advises on strategies for continual improvement of the QMS to enhance efficiency, effectiveness, and compliance with ISO 13485 requirements. This may involve analyzing data, identifying trends, and implementing process improvements.
- Post-Certification Support: Even after certification, we can continue to provide support and guidance to the organization to help maintain compliance with ISO 13485 requirements and address any challenges or issues that arise.
Overall, FDAmedix plays a crucial role in guiding our clients through the process of achieving and maintaining compliance with the ISO 13485 standard, ensuring that they establish and maintain effective quality management systems for medical devices.
Benefits of ISO 13485
Regulatory Compliance
ISO 13485 sets industry-specific standards for medical devices, focusing on critical areas like documentation and design control. Adhering to this standard helps organizations meet vital requirements, avoiding regulatory penalties and ensuring smoother operations in the medical device sector.
Increased Efficiency and Cost Savings
ISO 13485 implementation enables organizations in the medical device industry to streamline their operations and reduce inefficiencies, leading to significant cost savings. This enhanced efficiency not only optimizes resources but also strengthens their competitive edge in the global medical device marketplace.
Increased Customer Satisfaction
Implementing ISO 13485 showcases an organization’s commitment to high-quality medical devices and excellent customer service, enhancing customer satisfaction and loyalty. This leads to increased sales and market share, establishing the organization as a leader in the medical device industry.
Improved Product Quality and Safety
Implementing an ISO 13485-based Quality Management System helps ensure medical devices meet high safety and quality standards, reducing defects and enhancing patient outcomes. This approach minimizes recalls and boosts reliability, essential for medical device manufacturers aiming for excellence in patient care.