FDA Quality Management Systems (QMS) for Medical Device Manufacturers
FDAmedix helps small and mid-size medical device and IVD companies with the FDA Quality System Regulation (21 CFR Part 820). Our team can help you implement a new FDA-compliant QMS from the ground up or maintain your current compliance using FDA mock inspections and/or internal audits.
Overview of FDA QMSR
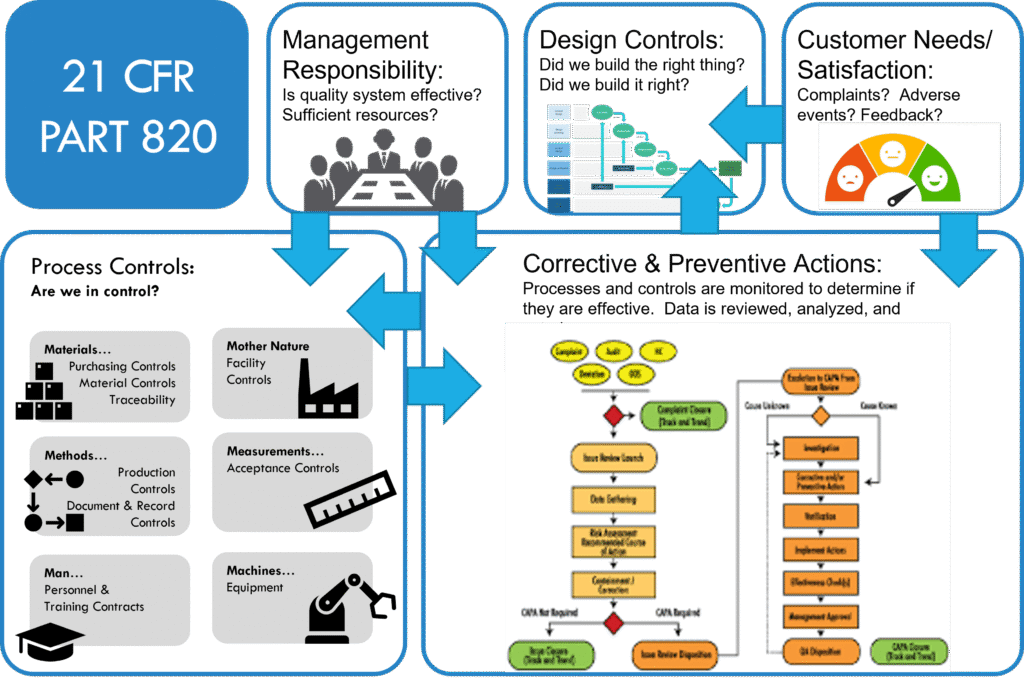
Final. Original text from the FDA Group (serach for 21 CFR 820). Modified using chatgpt.
FDA Introduces the Quality Management System Regulation (QMSR)
On February 2, 2024, the U.S. Food and Drug Administration (FDA) introduced the Quality Management System Regulation (QMSR), amending 21 CFR Part 820. This new rule incorporates ISO 13485:2016 standards, aiming to harmonize regulatory requirements and reduce the compliance burden for medical device manufacturers.
Streamlining and Simplifying Regulatory Oversight
The QMSR reflects the FDA’s commitment to simplifying and modernizing its regulatory framework. By creating a more concise and focused regulation, the agency seeks to enhance the efficiency of quality system oversight while maintaining its core mission of ensuring the safety and effectiveness of medical devices.
Integration of International Standards
Key elements of ISO 13485:2016, along with definitions from ISO 9000:2015, are incorporated by reference into the QMSR. This alignment promotes global consistency and clarity in quality system expectations.
Compliance Modifications and Documentation Updates
Although ISO 13485 and the previous 21 CFR Part 820 share many similarities, the QMSR includes specific modifications to fully meet FDA regulatory requirements. Device manufacturers may need to revise their documentation to align with QMSR expectations, particularly in areas such as risk management and compliance with electronic records and signature regulations.
Transition Timeline and Effective Date
To allow for a smooth shift, the FDA has provided a two-year transition period. The QMSR will officially take effect on February 2, 2026.
Inspections and Certification
The QMSR does not alter the FDA’s authority to conduct inspections. Moreover, certification to ISO 13485 will not exempt manufacturers from FDA inspections or compliance requirements.
Impact on Device Manufacturers
Manufacturers already compliant with ISO 13485:2016 are expected to experience minimal disruption. However, those currently aligned solely with the existing 21 CFR Part 820—particularly companies focused exclusively on the U.S. market—may need to undertake more substantial adjustments to meet the new requirements.
How FDAmedix Can Assist:
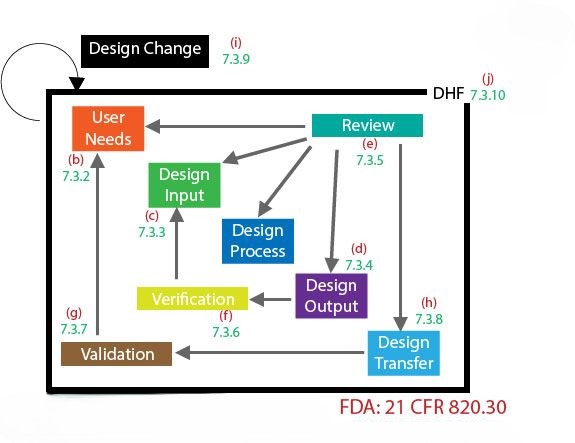
Final. Chatgpt was given a short list from FDA Group and asked to put some text in it.
Providing Gap Analysis for ISO 13485:2016 and FDA QMSR Compliance Gap
FDAmedix provides comprehensive gap assessments to evaluate your existing quality management system (QMS) against the ISO 13485:2016 standard and the newly introduced FDA QMSR. Our expert team identifies any discrepancies or areas of non-compliance, allowing you to address these issues before they impact your business operations or regulatory standing.
Assisting in the Creation of QMS Transition Plans and Closing Compliance Gaps
Navigating the changes between current FDA regulations and the new QMSR can be complex. FDAmedix works with your team to create customized transition plans, ensuring your QMS aligns seamlessly with both ISO 13485:2016 and the FDA’s updated regulations. We’ll help you prioritize gaps and develop strategies to close them efficiently.
Performing Audits and Mock Inspections to Ensure Inspection Preparedness
To prepare your team for FDA inspections, FDAmedix offers audits and mock inspections designed to test your compliance and readiness. Our experienced inspectors simulate FDA inspection processes, providing you with valuable insights and actionable feedback to ensure you’re well-prepared when the real inspection occurs.
Strengthening Risk Management Programs
Risk management is a key aspect of the QMSR and ISO 13485:2016, requiring thorough and systematic approaches to product risk analysis and mitigation. FDAmedix can review your current risk management practices, identify areas for improvement, and help develop enhanced strategies that align with both FDA requirements and international best practices.
Audit Suppliers to Evaluate Compliance with Quality Standards
Supplier quality is crucial to maintaining compliance with both FDA and ISO standards. FDAmedix offers auditing services to assess the quality compliance of your suppliers, ensuring that all components and materials meet regulatory requirements. This helps minimize the risk of non-compliance and ensures a consistent level of quality across your supply chain.