Medical Device CDMO and Critical Supplier QMS Audits
Virtually every medical device manufacturer depends on outside suppliers. Some provide critical components, others do final assembly. How do you know if their delivered products consistently meet your specifications? Does their QMS continue to function effectively? Will your quality management program withstand scrutiny from regulators? Supplier audits are the most efficient way to ensure that what you specified is what you received.
How We Help
Trust But Verify: Making Sure Your Suppliers Deliver What They Promised
You can outsource manufacturing but you can’t outsource your responsibility to comply with regulations. Even if your design and device manufacturing is 100% outsourced, it’s YOUR responsibility to ensure that your subcontractors are also complying with relevant medical device standards and regulations. This is both good business and a requirement of ISO 13485, FDA 21 CFR Part 820, and other international standards and regulations.
The good news is that you don’t have to do it alone. Many internal QMS teams simply don’t have the bandwidth to audit their suppliers on a regular basis and that’s where we can help. Our experienced QMS auditors can help you:
- Identify medical device CDMO strengths, weaknesses, nonconformities, and opportunities for improvement.
- Ensure that suppliers consistently comply with the requirements specified in supplier agreements and relevant standards or regulations.
- Increase efficiency and save money by addressing quality at the root (supplier) level and preventing problems from recurring.
- Develop a risk-based supplier audit program that supports your compliance but doesn’t weigh down your QA budget
Getting Your Non-Compliant Suppliers Into Compliance
Additionally, we can work with you to design a program to “develop” key suppliers that do not meet your requirements. This program can include the development of simple management systems or could include the implementation of robust improvement methodologies such as Lean or Six Sigma. For each supplier there can be a supplier development plan that is unique to the needs of the supplier and the critical nature of the parts being supplied.
Our Lead Auditors Are Located Worldwide
Regardless of where your suppliers or CDMO are located we’re ready to help. Our auditors are located throughout the US, Europe, Latin America and Asia, and specialize in ISO 9001, ISO 13485 and FDA QSR compliance. Our teams has performed hundreds of CDMO supplier audits for small medical device companies and multinationals alike.
We’re Ready to Help
Oriel STAT A MATRIX has worked with thousands of medical device and IVD manufacturers on supplier audits on supplier improvement programs. Let’s discuss your situation and see if we can assist.
Related Downloads
Provided below are a variety of practice related white papers, market updates and case studies.
Oriel STAT A MATRIX’s Goal
Help our life science customers meet regulatory requirements, boost efficiency, and improve patient outcomes SCHEDULE A FREE CONSULTATION
Get answers right now. Call

US OfficeWashington DC
EU OfficeCork, Ireland
Reliance on External Suppliers is a Given in Medical Device Manufacturing.
Ensuring that these suppliers deliver components, assemblies, and subsystems that meet your specifications is crucial for your success. How can you guarantee the effectiveness of their Quality Management Systems (QMS) and the integrity of your quality management program under regulatory scrutiny? The answer is simple: robust supplier audits. These audits are not just a formality; they are your strategic advantage, providing the assurance that your specifications are met consistently. By partnering with FDAmedix you can elevate your quality assurance processes, protect your brand, and maintain compliance, all while fostering a reliable supply chain that drives innovation and excellence.
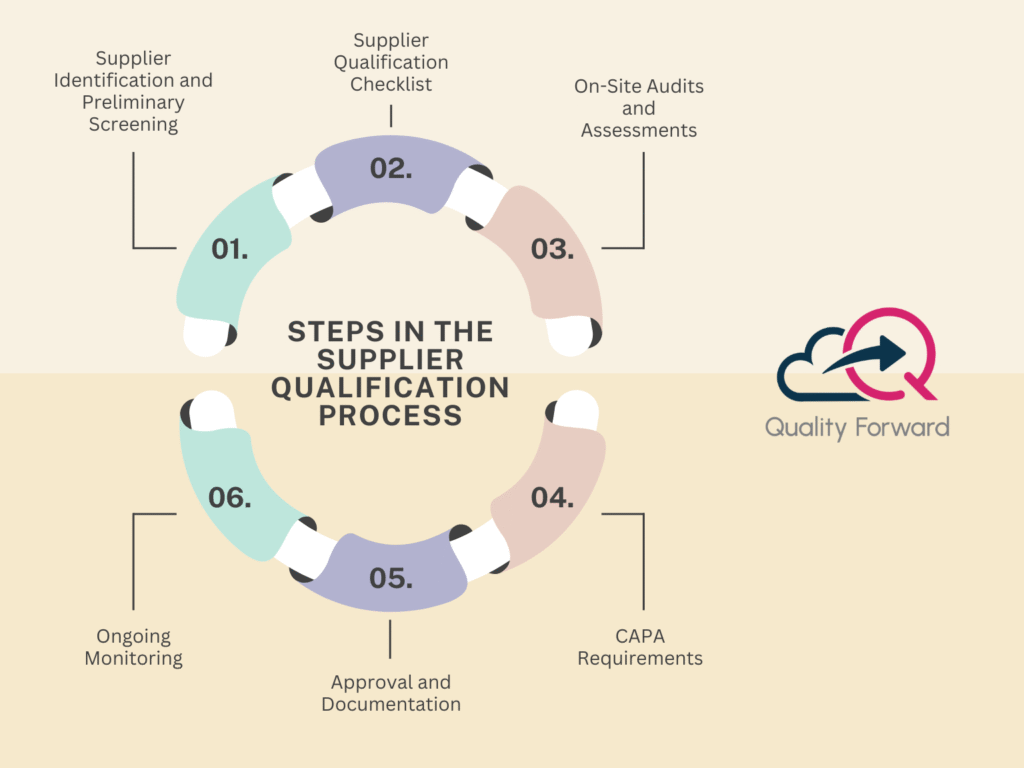

The Manufacturer Remains Responsible for Outsourced Parts and Assembly
Even when components or assembly processes are outsourced to third-party suppliers, the original manufacturer is accountable for ensuring that all parts meet regulatory standards and quality requirements.
That’s where FDAmedix comes in.
We’re your compliance co-pilot — helping medical device companies ensure their suppliers are compliant to ISO 13485, FDA 21 CFR Part 820, and supplier quality agreements through regular supplier audits, relieving your internal quality team to focus on internal matters.
Why FDAmedix Is Your Go-To for Supplier Quality Audits
Think of our QMS auditors as an extension of your team — but with sharper tools and deeper insights. When you partner with FDAMEDIX, you don’t just tick boxes. You get:
- A smart, risk-based supplier audit program that boosts compliance.
- A clear picture of supplier performance — from red flags to hidden strengths
- Confidence that your partners are living up to your quality agreements and regulatory obligations
- Time and cost savings by catching issues early — before they reach your product or your patients
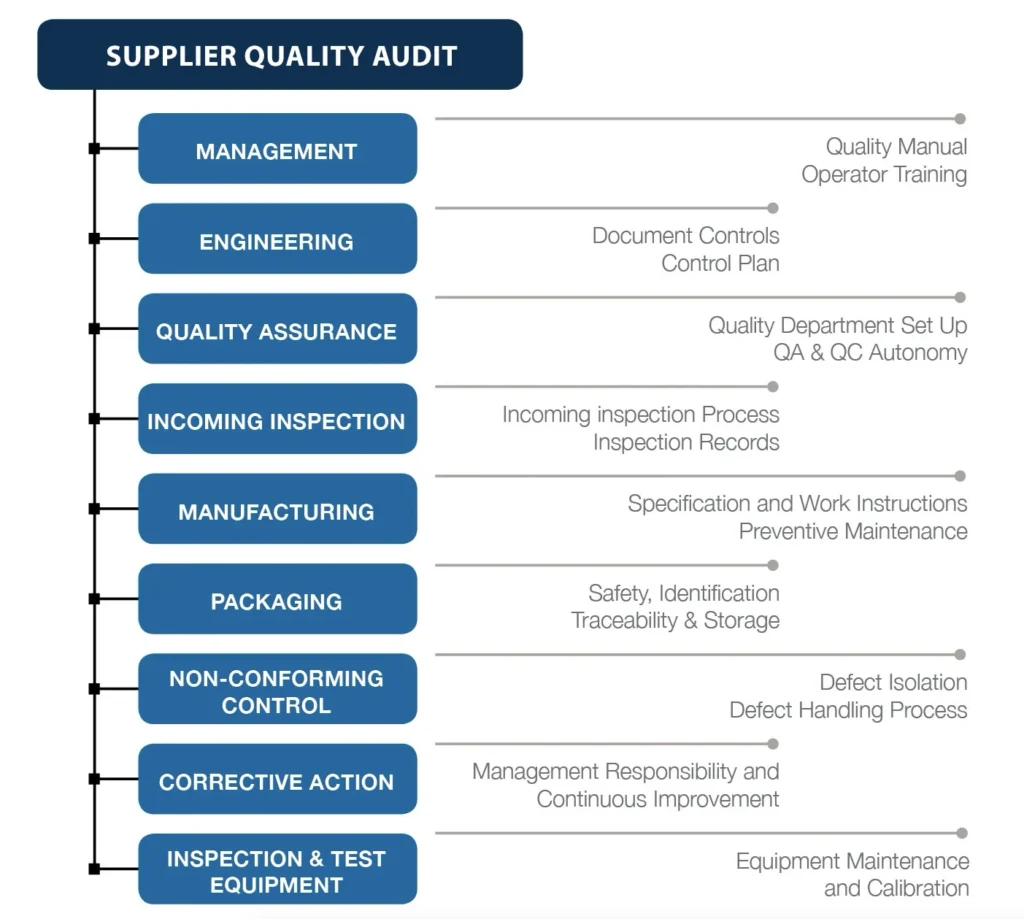
Don’t Just Audit — Elevate Your Suppliers
Not every supplier hits the mark. But that doesn’t mean you need to cut ties. FDAMEDIX helps you turn under performers into high-value partners.Beyond audits, FDAmedix offers tailored support to help suppliers implement or improve their Quality Management Systems (QMS), ensuring they align with your specific quality expectations. By partnering with us, you gain access to a wealth of knowledge and resources that empower your suppliers to achieve excellence, ultimately leading to superior products and increased customer satisfaction. Let FDAmedix be your trusted ally in elevating quality across your supply chain.
Global Reach, Local Expertise
Whether your suppliers are next door or halfway around the world, FDAMEDIX has you covered. Our lead auditors are strategically placed geographically bringing deep expertise in ISO 13485, FDA 21 CFR Part 820, IVDR, and MDR. We’ve run hundreds of supplier audits for small, midsize, large, startups, and medical device companies. Ready to see what we can do for you?