Why is Computer System Validation (CSV) important?
Computer System Validation (CSV) is a compliance requirement for all computerized systems operating in regulated environments. These systems must be validated to align with regulatory standards such as the U.S. FDA’s predicate rules (21 CFR Parts 210/211), EU GMP Annex 11 for computerized systems, and 21 CFR Part 11 for electronic records and signatures. The CSV process is guided by the GAMP® 5 risk-based approach and the V-model methodology. Validation must also confirm that the system is suitable for its intended use, including its architecture, operational environment, and scientific workflows or methods, to ensure regulatory compliance.
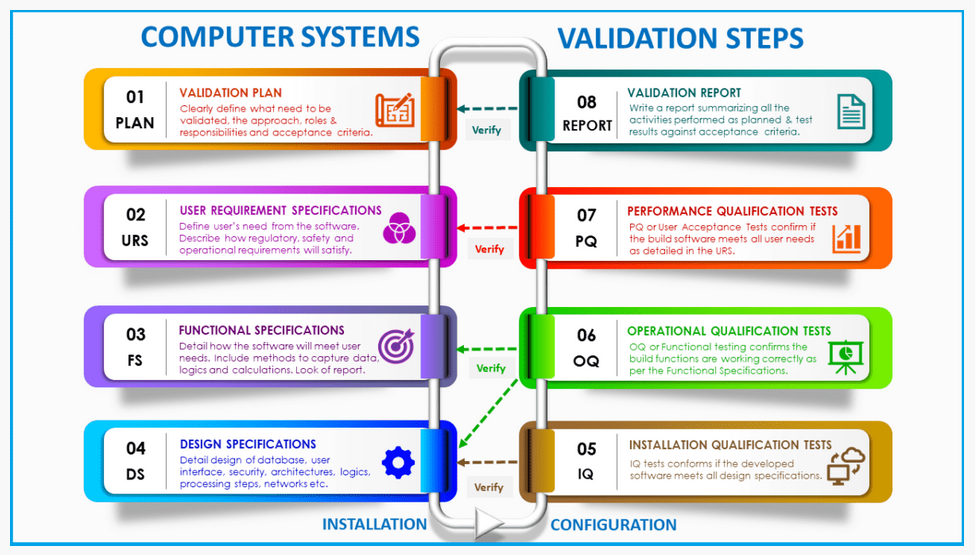
Transition from Computer Software Validation (CSV) to Computer Software Assurance (CSA)
In September 2022, the FDA released a new draft guidance titled “Computer Software Assurance for Production and Quality System Software.”
This long-anticipated document marks a significant evolution in how the FDA views software validation within the MedTech industry. It reflects a broader transition from the traditional Computer System Validation (CSV) model to a more modern, risk-based framework known as Computer Software Assurance (CSA).
Rather than applying a uniform validation approach to all systems, CSA emphasizes tailoring validation efforts based on the risk each system poses. While the underlying regulations remain unchanged, this shift encourages MedTech companies to adopt smarter, more efficient compliance strategies when validating the software tools used across their operations.
Why Choose FDAmedix for Computer System Validation/Assurance (CSV/CSA)?
Expertise in CSV/CSA Regulations and Best Practices
Our team brings deep expertise in the regulatory landscape of the life sciences industry, including FDA 21 CFR Part 11, EMA requirements, and GAMP 5 guidelines. We help you implement robust Computer System Validation (CSV) practices to ensure full compliance while upholding the highest standards for data integrity, reliability, safety, and security.
Comprehensive CSV Support from Start to Finish
We offer end-to-end CSV services—from initial planning through final validation. Our approach includes developing validation plans, executing testing protocols, and verifying system functionality to ensure your electronic systems operate consistently, securely, and in compliance with all relevant regulations.
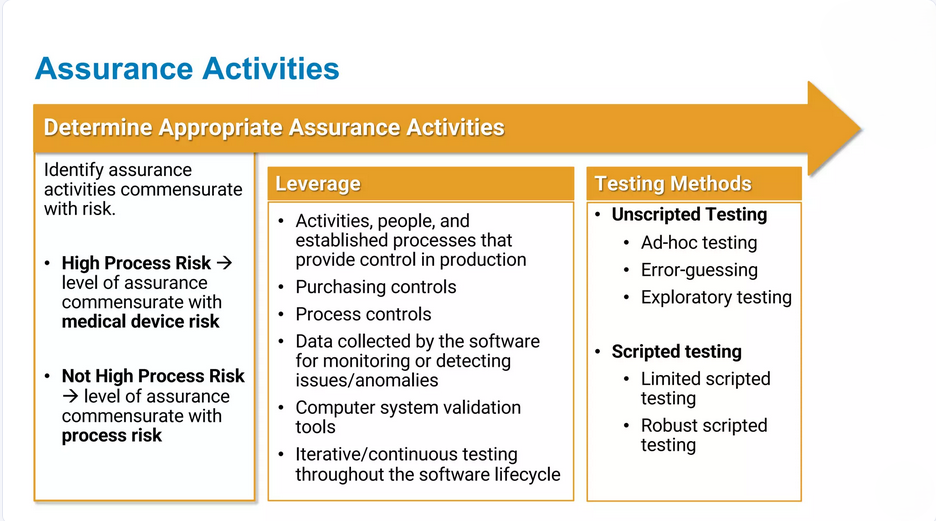
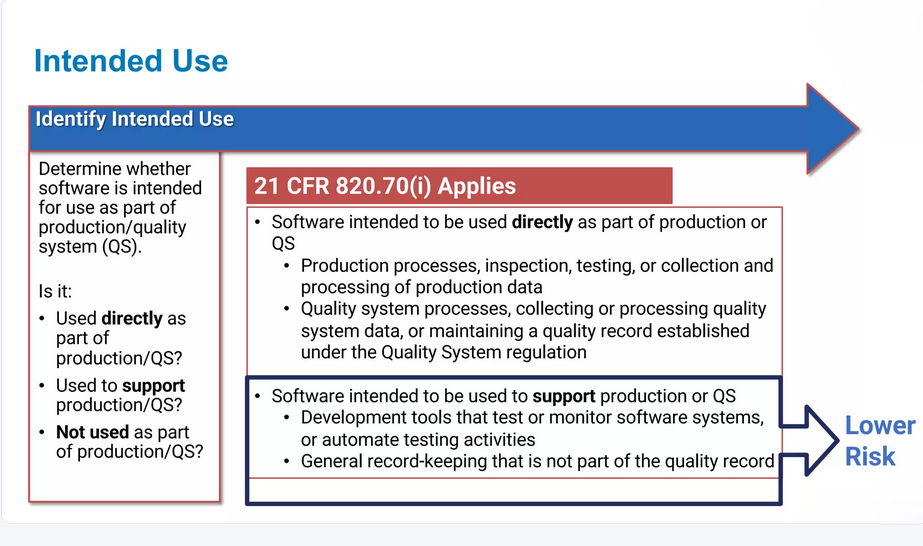
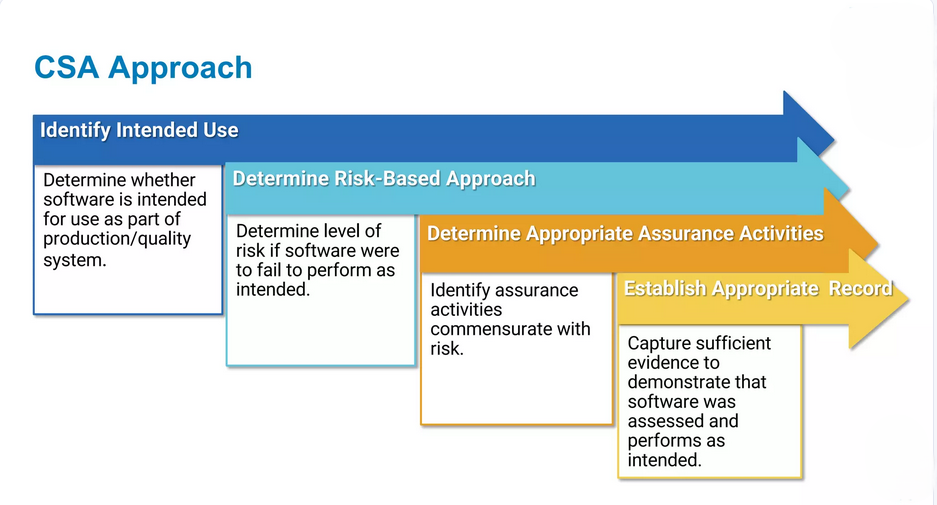
Tailored CSV Strategies
Recognizing that each system and organization is different, we provide customized validation strategies based on the specific complexity, business impact, and regulatory risk of your systems. Our tailored approach ensures your validation efforts are efficient, targeted, and effective.
Regulatory Compliance and Risk Mitigation
We help ensure your systems meet FDA, EMA, and other international regulatory standards—minimizing compliance risks and avoiding costly issues. Through comprehensive risk assessments, we identify vulnerabilities and implement controls to maintain secure and compliant systems.
Thorough Documentation and Audit Readiness
As part of the validation process, we deliver complete, detailed documentation to provide a clear audit trail of all validation activities. This ensures your organization is fully prepared for regulatory inspections and can readily demonstrate compliance with industry expectations.