Medical Device CAPA (Corrective and Preventive Action) Process Consultants
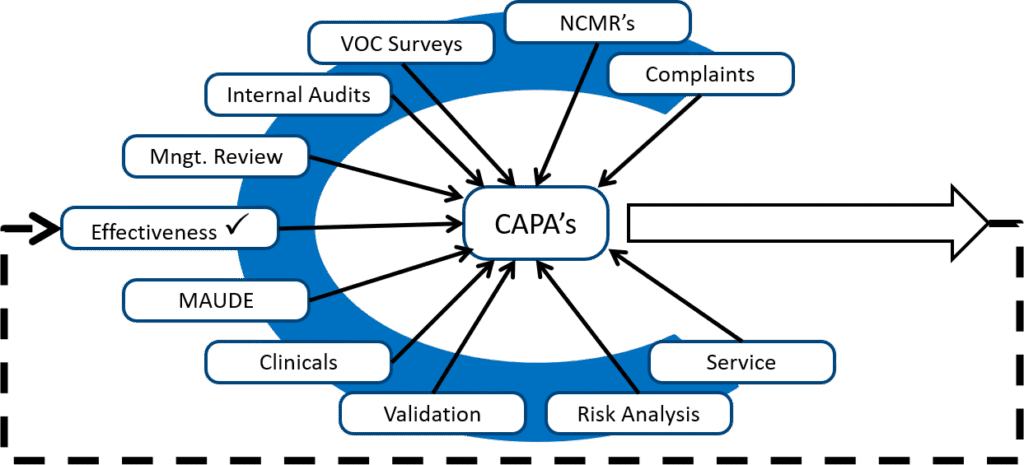
CAPA impacts every aspect of your quality management system, from processes to products. Medical device companies with effective CAPA programs identify problems, investigate their root causes, document related incidents, and refine their processes to ensure that shortcomings do not happen again. They also enjoy an added benefit beyond problem solving and better audit findings: Their CAPA processes yield data that can be used to improve operations and financial performance.
How We Help
Let us help assess and improve your current CAPA process and procedures
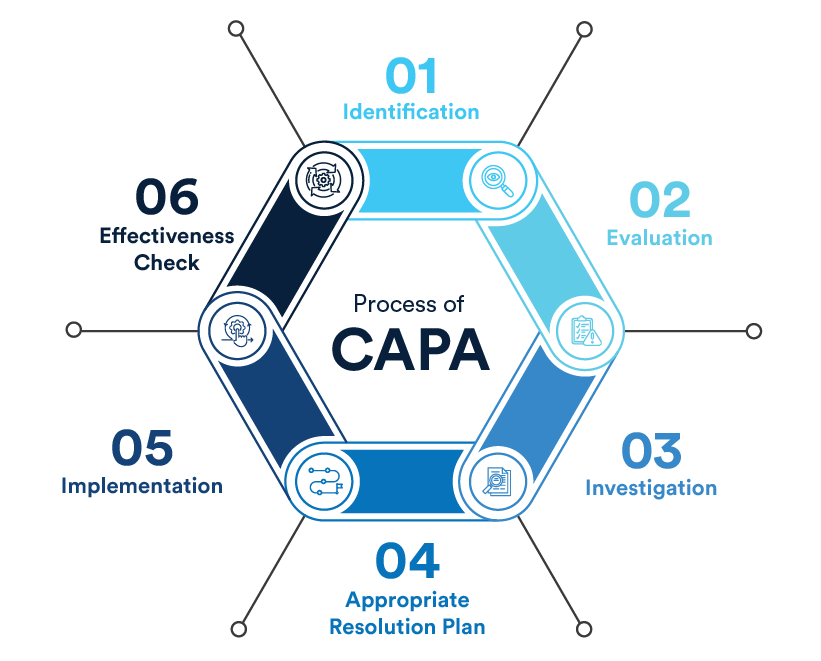
Oriel STAT A MATRIX consultants can conduct an independent review of your corrective and preventive action system that will uncover the areas in need of improvement. We can help you create and implement an action plan based on this assessment to ensure that your CAPA system is effective and efficient. We’ve been training and advising medical device companies on CAPA for decades, so we know how to apply best practices to your organization.
Ensuring you meet ISO and FDA CAPA quality requirements
Effective CAPA procedures can improve operational efficiency and product quality. As experienced medical device QA/RA consultants, we understand that maintaining regulatory compliance is also a key focus of CAPA. With this in mind, we can help you:
- Comply with the FDA regulations (21 CFR Part 820.100) and ISO 13485 requirements (sections 8.5.2 and 8.5.3).
- Reassure regulatory agencies that your company is capable of identifying and resolving issues.
- Generate objective evidence for regulators that your organization has corrected problems.
- Identify quality problems early in the cycle, before they lead to major nonconformities or an FDA 483 notice.
- Bring you back into compliance if a regulator or Notified Body audit leads to major findings.
Our team can conduct CAPA investigations for you
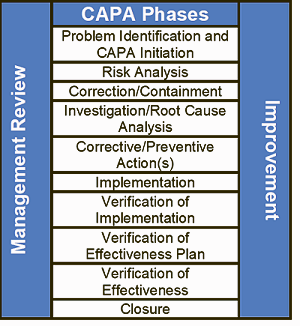
Many firms lack the time, resources, or internal skill sets to conduct effective CAPA investigations. Oriel STAT A MATRIX’s experts can plan and complete your CAPA investigations, so that your company obtains the results it needs to understand the root cause of a problem and take the needed corrective action. We will ensure that updated processes are validated and that all personnel impacted by those processes will be properly trained to maintain the improvements.
Contact us today to learn how we can help you improve your medical device CAPA process.
Related Downloads
Provided below are a variety of practice related white papers, market updates and case studies.
| Customized CAPA Training Addresses 483 Findings for a Medical Device Manufacturer | |
| Root Cause Analysis 101 |
Oriel STAT A MATRIX’s Goal
Help our life science customers meet regulatory requirements, boost efficiency, and improve patient outcomes REQUEST A PROPOSAL Or ask a question!
Get answers right now. Call

US OfficeWashington DC
EU OfficeCork, Ireland
Why CAPA Failures Keep Medical Device Companies in the FDA’s Crosshairs?
Year after year, “inadequate corrective and preventive action (CAPA) procedures” has been a persistent issue in the medical device industry, consistently appearing as one of the top concerns during FDA inspections. This persistent trend reveals a deeper issue: too many organizations are still struggling to build CAPA systems that not only pass inspections but actually work.
The consequences go far beyond warning letters and 483s. Undetected weaknesses in your CAPA program can quietly spread throughout your quality system—putting patients, products, and your reputation at risk.
We can evaluate your existing CAPA processes and strengthen them.
Third-party consultants regularly encounter a wide range of challenges in the field, which equips them with valuable experience and innovative approaches to problem-solving. This hands-on knowledge provides a unique perspective on what strategies are most effective in addressing specific issues or planning for future development.
For many small to mid-sized companies, employing full-time regulatory compliance professionals can be cost-prohibitive. FDAMedix consultants can perform an impartial assessment of your corrective and preventive action (CAPA) system to identify areas for improvement. We’ll help you develop and implement a strategic action plan to strengthen your CAPA system, ensuring it operates efficiently and meets regulatory expectations. Our team applies proven best practices tailored to fit your organization’s specific needs.
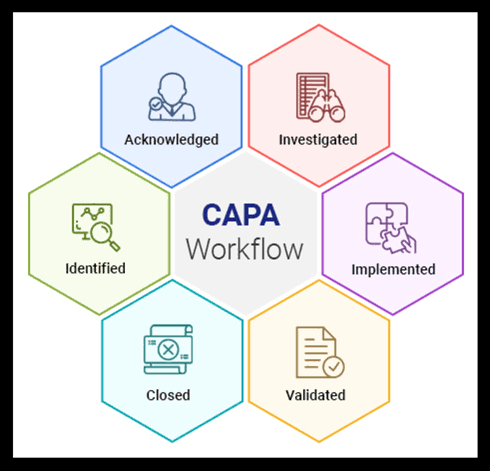
Main CAPA Challenges
Common reasons manufacturers struggle with CAPA include:
Reactive mindset: Many assume preventive actions only follow a corrective action: first a nonconformity occurs, then corrective steps are taken, and only afterward are preventive measures considered. This reactive stance weakens CAPA. A proactive system that identifies risks before they become nonconformities is far more effective.
Poor cross‑functional collaboration: CAPA is often managed by quality alone. Without strong communication and involvement from other departments, CAPA efforts frequently fail to produce meaningful change.
Inadequate root cause identification: CAPA and root cause analysis go hand in hand. Organizations sometimes spend effort on CAPA activities but neglect to properly determine underlying causes, which undermines both correction and prevention.
Other recurring issues: Lacking a documented CAPA process altogether; not following their own CAPA procedures; having CAPA processes that don’t meet regulatory requirements.
We can manage CAPA investigations on your behalf.
Many organizations struggle to dedicate the time, resources, or specialized expertise required for thorough and effective CAPA (Corrective and Preventive Action) investigations. FDAmedix experienced team can manage the entire CAPA process for you—from root cause analysis to implementing corrective actions—ensuring your organization meets compliance requirements and drives meaningful improvement.
We take a comprehensive approach, validating updated processes and ensuring all affected personnel receive the necessary training to sustain long-term changes. With our support, you can strengthen your quality system and reduce the risk of recurring issues.
Reach out today to discover how we can help you enhance your medical device CAPA process and maintain regulatory confidence. (Note: MA – call to action)